1. Course Identification
| Course | Practice Basic Pharmaceutical Chemistry | |||
| Faculty | Mathematics and Natural Sciences | Study Program | Pharmacy | |
| Code | SFA-111 | Credits | 1 credits | |
| Group | Study Program | Status | Compulsory | |
| Semester | I (First) | Availability | Limited in Study Program | |
| Learning Method | Practice | Media | online | |
| Cluster | Biomedical and Pharmaceutical Sciences: Pharmaceutical Chemistry | Prerequisite | – | |
2. Course Description
Mata kuliah Praktikum Kimia Farmasi Dasar dalam Kurikulum Farmasi 2017 diberikan kepada mahasiswa semester I (satu) dengan bobot 1 sks. Mata kuliah ini merupakan mata kuliah wajib yang dapat ditempuh tanpa prasyarat dan bertujuan untuk mendukung Capaian Pembelajaran Lulusan berupa kemampuan bersikap dan bekerja mandiri maupun berkelompok secara professional dan etis dengan tetap memperhatikan aspek keselamatan dan kesehatan kerja di laboratorium, serta dapat mengaplikasikan teknik dasar laboratorium dan kimia dasar untuk analisis kualitas bahan baku obat, pangan, dan kosmetik. Pada mata kuliah ini mahasiswa akan mempraktikkan teknik ketrampilan dasar laboratorium, seperti penggunaan alat gelas dan alat ukur, cara menimbang, membuat larutan dengan berbagai satuan konsentrasi, cara pengenceran, pembakuan larutan, membuat larutan buffer dengan benar; mengamati perubahan pH; mengamati reaksi kimia dan menghitung persen hasil; menentukan berat molekul senyawa berdasarkan sifat fisik larutan; teknik titrasi asam-basa; menggambar struktur kimia menggunakan chemical drawing software, serta mengevaluasi kualitas bahan baku obat menurut kompendia.
1. Learning Outcome
| LO Code | LO |
| SIF3 | Able to carefully and thoroughly apply responsible attitude, dedication and punctuality based on sincerity, honesty and integrity at work along with the ability to show bravery in expressing the truth whilst maintaining politeness, ethics and affection and promoting mutual benefit. |
| KUF4 | Able to apply the concept of work health and safety while carrying out pharmaceutical jobs. |
| KKF4 | Able to apply standardized analytical methods for the quality and halal assurance of drugs and cosmetics. |
2. Course Learning Outcome and indicators
| Supported LO Code | CLO Code | CLO and indicators |
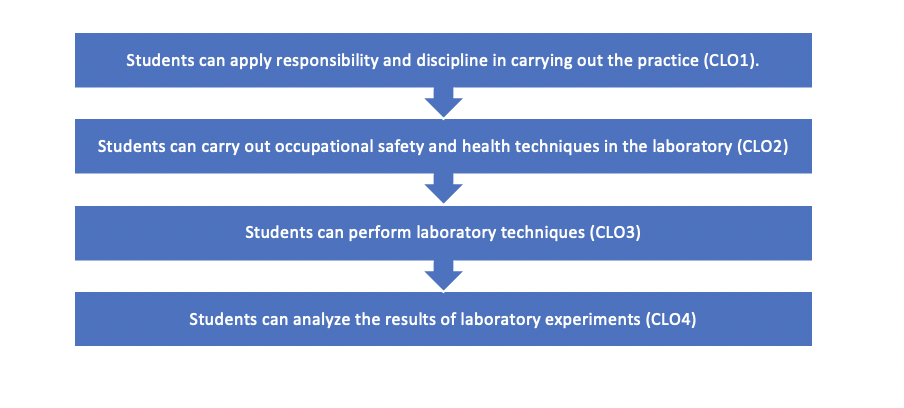
| SIF3 | CLO1 | Students can apply responsibility and discipline in carrying out the practice.
1. Students can participate in a whole series of practical activities properly. 2. Students can collect worksheet reports on time. |
| KUF4 | CLO2 | Students can carry out occupational safety and health techniques in the laboratory.
1. Students understand the safety lab application in all experimental sessions. 2. Students can recognize safety signs, MSDS, chemical labels properly. |
| KKF4 | CLO3 | Students can perform laboratory techniques.
1. Students understand the experimental work procedure correctly. 2. Students document the process and work results properly.. |
| CLO4 | Students can analyze the results of laboratory experiments.
1. Students can process the observed data correctly. 2. Students can present the results of data processing in the form of a discussion on the worksheet properly. 3. Students can calculate the RSD and RPD scores correctly. 4. Students can calculate the percent of the result of compound synthesis reactions. 5. Students can recognize the qualitative test (identification) of synthesized compounds. 6. Students can recognize the contents of Indonesian Pharmacopeia V Edition correctly. |
3. Course Learning Outcome Map Analysis
Primary References
- Chang, R., 2003, Kimia Dasar: Konsep-konsep Kimia Inti, 3rd edition, Jilid 1 dan 2, diterjemahkan oleh Muhammad A.M., et al., Penerbit Erlangga, Jakarta.
- Anonim, 2014, Farmakope Indonesia edisi V, Kementrian Kesehatan Republik Indonesia, Jakarta.
- Langsjoen, A., Everett, G.W., Lieder, P., Lata, A.J., 1988, Experiments in General, Organic and Biological Chemistry, Harcout Brace, Jovanovich Publisher, Orlando, Florida, 17-89
- Timberlake, K.C., 2006, Chemistry, An Introduction to General, Organic, And Biological Chemistry, 9th edition, Pearson Education Inc., USA.
- Murov, S., Stedjee, B., 2004, Experiments and Exercises in Basic Chemistry, 6th Ed., Wiley, New York.
- Coyne, G.S., 2006, The Laboratory Companion: A Practical Guide to Materials, Equipment, and Technique, Revised Ed., Wiley interscience, New Jersey.
- Slowinski E. J., Wolsey W.C., Rossi R.C., 2016, Chemical Principles in the Laboratory, Eleventh Ed., Cengage Learning, USA.
1. Learning activities
| Sesion | CLO | Topic | Learning activities |
| 1 | CLO1
CLO2 |
Study contract, safety laboratory and general introduction to basic laboratory techniques | 1. Students recognize MK learning outcomes, practical material summary, assessment system, references, and class rules (100 minutes). Lecturers deliver material using the TMD method.
2. Students understand videos about safety laboratories (chemical safety, MSDS, and material handling) (30 minutes). Lecturer uploads video (ASM) 3. Students understand general videos of basic laboratory techniques (30 minutes). Lecturer uploads video (ASM) 4. Students work on multiple-choice questions about safety lab and basic laboratory techniques (10 minutes). Lecturers make questions using google form (ASM) |
| 2 | CLO1
CLO4 |
Introduction of compendia and preparation of test solutions | 1. Students recognize the information listed and how to find it from the FI compendia (50 minutes). Lecturers deliver material using the TMD method.
2. Students calculate how to make the test solution from the compendia (60 minutes). Lecturers deliver material using the TMD method. 3. Students do the task of finding and writing information from FI (30 minutes). Lecturers make questions using google classroom (ASM) 4. Students do the task of calculating the preparation of test solutions from FI (30 minutes). Lecturers make questions using google classroom (ASM) |
| 3 | CLO1
CLO3 |
Marvinskecth | 1. Students recognize Marvin sketch software features (50 minutes). Lecturer uploads video (ASM)
2. Students operate the Marvin sketch software for drawing and naming the chemical structure of medicine (100 minutes). ASM 3. Students create and record work results, then upload them to Google Classroom. (15 minutes). ASM |
| 4 | CLO1
CLO3 CLO4 |
Basic lab techniques 1 (weighing, solution preparation, and dilution, weighing solids) | 1. Students understand the experimental video (60 minutes). Video uploaded before practice session. Students complete worksheets (ASM)
2. Students recognize the purpose and basis of the experimental theory (10 minutes). Lecturers deliver material using the TMD method. 3. Students study the experimental video. A discussion was held with an assistant in the breakroom (60 minutes). TMD 4. Students work on multiple-choice questions post-test (10 minutes). TMD 5. Students conclude the experiment video and document it in a worksheet (80 minutes). ASM |
| 5 | CLO1
CLO3 CLO4 |
Data processing | 1. Students recognize how to process data (calculating average value, standard deviation, relative standard deviation, relative percent of the difference, linear regression) using a calculator, and Microsoft Office Excel (90 minutes). Lecturer uploads video (ASM)
2. Students complete the data processing calculation task (60 minutes). ASM 1. Students create and record work results, then upload them to Google Classroom. (15 minutes). ASM |
| 6 | CLO1
CLO2 CLO3 CLO4 |
Basic laboratory techniques 2 (buffering, weighing semisolid and liquid preparations), using a pH meter. | 1. Students understand the experimental video (60 minutes). Video uploaded before practice session. Students complete worksheets (ASM)
2. Students recognize the purpose and basis of the experimental theory (10 minutes). Lecturers deliver material using the TMD method. 3. Students study the experimental video. A discussion was held with an assistant in the breakroom (60 minutes). TMD 4. Students work on multiple-choice questions post-test (10 minutes). TMD 1. Students conclude the experiment video and document it in a worksheet (80 minutes). ASM |
| 7 | CLO1
CLO2 CLO3 CLO4 |
Chemical reactions (precipitation and redox) | 1. Students understand the experimental video (60 minutes). Video uploaded before practice session. Students complete worksheets (ASM)
2. Students recognize the purpose and basis of the experimental theory (10 minutes). Lecturers deliver material using the TMD method. 3. Students study the experimental video. A discussion was held with an assistant in the breakroom (60 minutes). TMD 4. Students work on multiple-choice questions post-test (10 minutes). TMD 1. Students conclude the experiment video and document it in a worksheet (80 minutes). ASM |
| 8 | CLO1
CLO2 CLO3 CLO4 |
Stoichiometry 1 (synthesis of formic acid by distillation method) | 1. Students understand the experimental video (60 minutes). Video uploaded before practice session. Students complete worksheets (ASM)
2. Students recognize the purpose and basis of the experimental theory (10 minutes). Lecturers deliver material using the TMD method. 3. Students study the experimental video. A discussion was held with an assistant in the breakroom (60 minutes). TMD 4. Students work on multiple-choice questions post-test (10 minutes). TMD Students conclude the experiment video and document it in a worksheet (80 minutes). ASM |
| 9 | CLO1
CLO3 CLO4 |
Stoichiometry 2 (preparation of methyl salicylate and reflux method) | 1. Students understand the experimental video (60 minutes). Video uploaded before practice session. Students complete worksheets (ASM)
2. Students recognize the purpose and basis of the experimental theory (10 minutes). Lecturers deliver material using the ASM method. 3. Students work on multiple-choice questions post-test (10 minutes). ASM 4. Students study the experimental video. Discussions were held among group members (60 minutes). ASK 1. Students conclude the experiment video and document it in a worksheet (80 minutes). ASM |
| 10 | CLO1
CLO2 CLO3 CLO4 |
Colligative properties of solutions (freezing and boiling points) | 1. Students understand the experimental video (60 minutes). Video uploaded before practice session. Students complete worksheets (ASM)
2. Students recognize the purpose and basis of the experimental theory (10 minutes). Lecturers deliver material using the TMD method. 3. Students study the experimental video. A discussion was held with an assistant in the breakroom (60 minutes). TMD 4. Students work on multiple-choice questions post-test (10 minutes). TMD 1. Students conclude the experiment video and document it in a worksheet (80 minutes). ASM |
| 11 | CLO1
CLO3 CLO4 |
Determination of Salicylic Acid by Titration Method | 1. Students understand the experimental video (60 minutes). Video uploaded before practice session. Students complete worksheets (ASM)
2. Students recognize the purpose and basis of the experimental theory (10 minutes). Lecturers deliver material using the ASM method. 3. Students work on multiple-choice questions post-test (10 minutes). ASM 4. Students study the experimental video. Discussions were held among group members (60 minutes). ASK 1. Students conclude the experiment video and document it in a worksheet (80 minutes). ASM |
| 12 | CLO1
CLO2 CLO3 CLO4 |
Reaction kinetics (influence of temperature, reactant concentration, catalyst) | 1. Students understand the experimental video (60 minutes). Video uploaded before practice session. Students complete worksheets (ASM)
2. Students recognize the purpose and basis of the experimental theory (10 minutes). Lecturers deliver material using the TMD method. 3. Students study the experimental video. A discussion was held with an assistant in the breakroom (60 minutes). TMD 4. Students work on multiple-choice questions post-test (10 minutes). TMD 1. Students conclude the experiment video and document it in a worksheet (80 minutes). ASM |
| 13 | CLO1
CLO2 CLO3 CLO4 |
Separation of compound mixtures (distribution coefficient) | 1. Students understand the experimental video (60 minutes). Video uploaded before practice session. Students complete worksheets (ASM)
2. Students recognize the purpose and basis of the experimental theory (10 minutes). Lecturers deliver material using the TMD method. 3. Students study the experimental video. A discussion was held with an assistant in the breakroom (60 minutes). TMD 4. Students work on multiple-choice questions post-test (10 minutes). TMD 1. Students conclude the experiment video and document it in a worksheet (80 minutes). ASM |
| 14 | CLO1
CLO2 CLO3 CLO4 |
Final exam | Students do written questions (100 minutes). TMD |
2. Learning Experience
Students attend lectures online synchronously or asynchronously using google classroom media, zoom, panopto, google form, google slides.
1. Assessment
| CLO Code | Assessment | Weight |
| CLO1 | Assessment using a rubric, accuracy of attendance, presentation of worksheets, and timeliness of submitting assignments/worksheets/tests. | 15% |
| CLO2 | Online safety lab test assessment. | 15% |
| CLO3 | Assessment is based on daily observations using a rubric, worksheets, and exams. | 35% |
| CLO4 | Assessment of worksheets, assignments, tests, exams. | 35% |
2. Grading and Evaluation Systems
Grading system uses reference assessment as follows:
| Letter Value | Weight | Minimum Value | Value Range |
| A | 4 | 80 | 80.00 – 100 |
| A- | 3.75 | 77.5 | 77.50 – 79.99 |
| A/B | 3.5 | 75 | 75.00 – 77.49 |
| B+ | 3.25 | 72.5 | 72.50 – 74.99 |
| B | 3 | 70 | 70.00 – 72.49 |
| B- | 2.75 | 67.5 | 67.50 – 69.99 |
| B/C | 2.5 | 65 | 65.00 – 67.49 |
| C+ | 2.25 | 62.5 | 62.50 – 64.99 |
| C | 2 | 60 | 60.00 – 62.49 |
| C- | 1.75 | 55 | 55.00 – 59.99 |
| C/D | 1.5 | 50 | 50.00 – 54.99 |
| D+ | 1.25 | 45 | 45.00 – 49.99 |
| D | 1 | 40 | 40.00 – 44.99 |
| E | 0 | 0 | < 40 |
Evaluation Systems
Each student must score at least 60 for the CLO average. If it has not fulfilled it, the student must undertake a repair assignment for the related CLO, except for CLO1.
