1. Course Identification
| Course | Basic Pharmaceutical Chemistry | |||
| Faculty | Mathematics and Natural Sciences | Study Program | Pharmacy | |
| Code | SFA-107 | Credits | 2 credits | |
| Group | Study Program | Status | Compulsory | |
| Semester | I (First) | Availability | Limited in Study Program | |
| Learning Method | Class | Media | online | |
| Cluster | Biomedical and Pharmaceutical Sciences: Pharmaceutical Chemistry | Prerequisite | – | |
2. Course Description
Basic Pharmacy Chemistry course in Pharmacy Curriculum is given to first-semester students with a weight of 2 credits. This course is a compulsory subject that can be taken without preconditions and aims to support Graduate Learning Outcomes in the mastery of basic chemistry, which will be used in pharmaceutical work professionally and ethically. In this course, students will study the physicochemical properties of compounds, stoichiometry, chemical reactions (acid/base, redox), reaction kinetics, and the colligative properties of solutions. After understanding the basic knowledge of chemistry, students will learn about applying this basic knowledge to pharmaceutical work.
1. Learning Outcome
| LO Code | LO |
| SIF3 | Able to carefully and thoroughly apply responsible attitude, dedication and punctuality based on sincerity, honesty and integrity at work along with the ability to show bravery in expressing the truth whilst maintaining politeness, ethics and affection and promoting mutual benefit. |
| PEF1 | Able to comprehend the theories, methods and concepts within the scope of pharmaceutical sciences and technologies in the disciplines of pharmaceutics, pharmacognosy, pharmaceutical chemistry and pharmacology as well as their application in pharmaceutical works. |
2. Course Learning Outcome and indicators
| Supported LO Code | CLO Code | CLO and indicators |
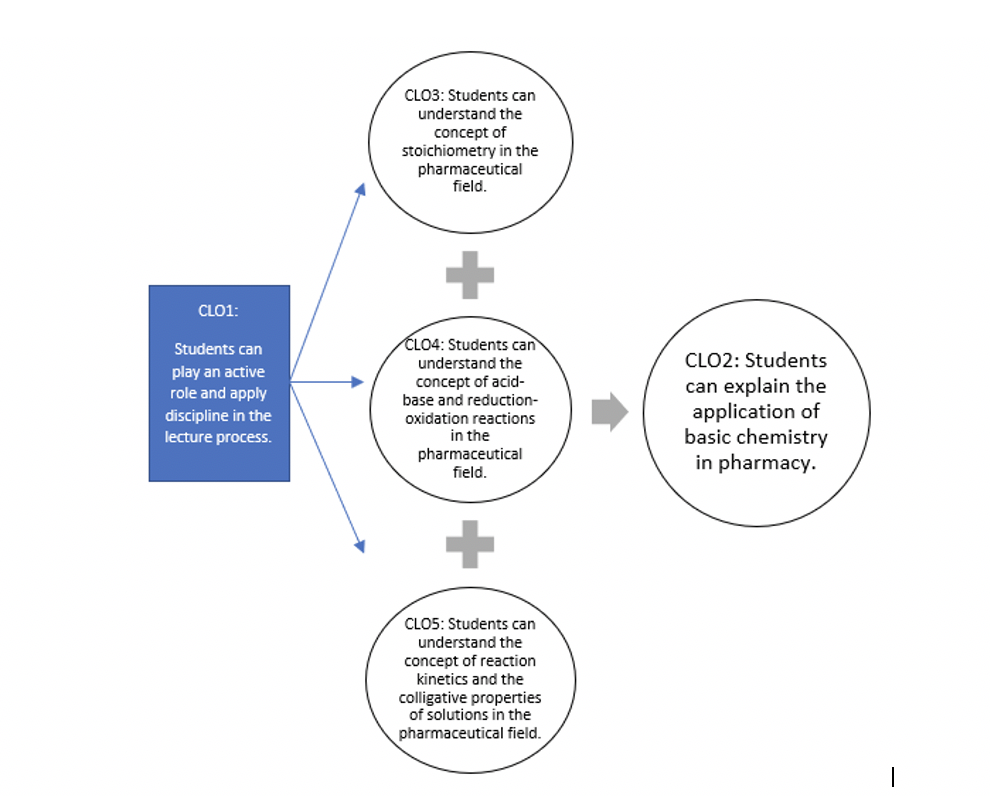
| SIF3 | CLO1 | Students can play an active role and apply discipline in the lecture process.
1. Students can participate in a whole series of lecture activities correctly. 2. Students can submit assignments on time. 3. Students can present and present assignments, as well. |
| PEF1 | CLO2 | Students can explain the application of basic chemistry in pharmacy.
1. Students can explain the physicochemical properties of medicinal compounds well. 2. Students can give examples of the role of physicochemical properties of drugs in the pharmaceutical field properly. |
| CLO3 | Students can understand the concept of stoichiometry in the pharmaceutical field.
1. Students can solve problems related to stoichiometry correctly. 2. Students can calculate the correct preparation of solutions with various concentration units. |
|
| CLO4 | Students can understand the concept of acid-base and reduction-oxidation reactions in the pharmaceutical field.
1. Students can determine the acid-base properties of drugs, acid-base strength, pH of solutions, salt hydrolysis, and ionization of medicinal compounds. 2. Students can count the amount of weak ash and salt in buffering correctly. 3. Students can write down and balance redox reactions correctly. 4. Students can solve the stoichiometric problems of redox reactions correctly. |
|
| CLO5 | Students can understand the concept of reaction kinetics and the colligative properties of solutions in the pharmaceutical field.
1. Students can calculate the reaction rate and determine the order of chemical reactions correctly. 2. Students can calculate the correct half-life and expiration of drugs. 3. Students can calculate mole fraction, molality, lowering freezing point, boiling point increase, and osmotic pressure correctly. 4. Students can calculate the molecular weight of a compound using the colligative properties of the solution correctly. |
3. Course Learning Outcome Map Analysis
- Chang, R., 2003, Kimia Dasar: Konsep-konsep Kimia Inti, 3rd edition, Jilid 1 dan 2, diterjemahkan oleh Muhammad A.M., et al., Penerbit Erlangga, Jakarta.
- Cairns, D., 2008, Essentials Pharmaceutical Chemistry 3rd edition, Pharmaceutical Press, London.
- Harris, D. C., 2010, Quantitative Chemical Analysis Eighth Edition, W. H. Freeman and Company, New York.
- Reddy I. K., dan Khan M. A., 2004, Essential Math and Calculation for Pharmacy technicians, CRC Press, Boca Raton, Florida.
- Timberlake, K.C., 2015, Chemistry, An Introduction to General, Organic, and Biological Chemistry, 12th edition, Pearson Education Inc., USA.
- Sarker S.D., dan Nahar L., 2007, Chemistry for Pharmacy Students, John Wiley & Sons, Ltd., England.
- Beale J. M., Jr., dan Block J. H., 2011, Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, twelfth edition, Lippincott Williams & Wilkins, Philadelphia
1. Learning activities
| Sesion | CLO | Topic | Learning activities |
| 1 | CLO1 | Study contracts; Chemistry in pharmacy | 1. Students are familiar with course concept maps, material summaries, grading systems, references, and class rules (50 minutes).
2. Students know examples of the scope of pharmaceutical work (designing drug dosage forms, drug stability, drug pharmacology in general) from a chemical point of view (40 minutes) 3. Students recognize the compendia (10 minutes). |
| 2 | CLO1
CLO2 |
Physicochemical properties of active pharmaceutical ingredient | 1. Students know the physicochemical properties of medicinal compounds that play a role in drug development and influence the pharmacological effects, such as describing the APIs, melting point, partition coefficient, and polarity (40 minutes).
2. Students understand the physicochemical properties of medicinal compounds, the dissociation coefficient, solubility, Lipinski rule of five (50 minutes). 3. Students take a quiz (10 minutes) |
| 3 | CLO1
CLO4 |
Acid-base properties, Salt hydrolysis | 1. Students understand acid-base theory, acid-base properties of water, ionic product, ionization constant, salt hydrolysis (50 minutes).
2. Students calculate the pH of the solution, calculate the pH of salt (50 minutes). 3. Students do assignments independently (60 minutes). |
| 4 | CLO1
CLO4 |
Buffering and ionization of API | 1. Students understand a video about the Handerson-Hasselbach equation in making buffer solutions, buffer applications in the pharmaceutical field, ionization of medicinal compounds (100 minutes)
2. Students do assignments independently (60 minutes). |
| 5 | CLO1
CLO4 |
Acid / base properties of API and liquid-liquid extraction | 1. Students understand acid/base groups, pKa and pKb values of medicinal compounds, acid/base applications in liquid-liquid extraction techniques (90 minutes).
2. Students take a quiz (10 minutes) |
| 6 | CLO1
CLO3 |
Stoichiometry | 1. Students understand videos about moles, reagent coefficients, empirical and molecular formulas, limiting reagents, percent yield (80 minutes).
2. Students take a quiz (20 minutes). 3. Students do assignments independently (60 minutes). |
| 7 | CLO1
CLO3 |
Solution Stoichiometry | 1. Students understand chemical reactions in an aqueous medium, acid-base reactions, acid-base titration (50 minutes)
2. Students calculate the analyte in sample (50 minutes). 3. Students do assignments independently (60 minutes). |
| 8 | CLO2
CLO3 CLO4 |
Midterm exam | Students do written questions (100 minutes). |
| 9 | CLO1
CLO4 |
Reduction and oxidation reactions | 1. Students understand how to determine oxidation states, balance redox reactions with the half-reaction method, stoichiometric questions using redox reactions (100 minutes)
2. Students do assignments independently (60 minutes). |
| 10 | CLO1
CLO4 |
Reduction and oxidation reactions | 1. Students understand redox reaction stoichiometry (100 minutes).
2. Students do assignments independently (60 minutes). |
| 11 | CLO1
CLO5 |
Kinetics of reaction | 1. Students understand videos about reaction rates, rate laws, reaction order determination, the relationship between reactant concentration and time (80 minutes).
2. Students take a quiz (20 minutes). |
| 12 | CLO1
CLO5 |
Kinetics of reaction | 1. Students understand the activation energy and rate constants’ dependence on temperature, catalyst, and drug stability (100 minutes).
2. Students do assignments independently (60 minutes). |
| 13 | CLO1
CLO5 |
Physical properties of the solution | 1. Students understand videos about decreasing vapor pressure, increasing boiling point, decreasing vapor pressure, osmotic (80 minutes).
2. Students take a quiz (20 minutes). |
| 14 | CLO1
CLO5 |
Physical properties of the solution | 1. Students understand the colligative properties of electrolyte solutions, determination of molecular weight using the solution’s colligative properties (100 minutes).
2. Students do assignments independently (60 minutes). |
| 15 | CLO1
CLO2 |
Chemistry in pharmacy word | 1. Students in groups make presentations on “Chemistry in Pharmacy” using google slides (100 minutes).
2. Student groups present their paper (100 minutes). |
| 16 | CLO2
CLO5 |
Final exam | Students do written questions (100 minutes). |
2. Learning Experience
Students attend lectures online synchronously or asynchronously using google classroom media, zoom, panopto, google form, google slides
1. Assessment
| CLO Code | Assessment | Weight |
| CLO1 | Assessment based on attendance, assignment, presentation, and timeliness of assignment submission. | 10% |
| CLO2 | Assessment is based on the results of written examinations and assignments. | 15% |
| CLO3 | Assessment is based on the results of written examinations and assignments. | 20% |
| CLO4 | Assessment is based on the results of written examinations and assignments. | 22.5% |
| CLO5 | Assessment is based on the results of written examinations and assignments. | 22.5% |
2. Grading and Evaluation Systems
Grading system uses reference assessment as follows:
| Letter Value | Weight | Minimum Value | Value Range |
| A | 4 | 80 | 80.00 – 100 |
| A- | 3.75 | 77.5 | 77.50 – 79.99 |
| A/B | 3.5 | 75 | 75.00 – 77.49 |
| B+ | 3.25 | 72.5 | 72.50 – 74.99 |
| B | 3 | 70 | 70.00 – 72.49 |
| B- | 2.75 | 67.5 | 67.50 – 69.99 |
| B/C | 2.5 | 65 | 65.00 – 67.49 |
| C+ | 2.25 | 62.5 | 62.50 – 64.99 |
| C | 2 | 60 | 60.00 – 62.49 |
| C- | 1.75 | 55 | 55.00 – 59.99 |
| C/D | 1.5 | 50 | 50.00 – 54.99 |
| D+ | 1.25 | 45 | 45.00 – 49.99 |
| D | 1 | 40 | 40.00 – 44.99 |
| E | 0 | 0 | < 40 |
Evaluation Systems
Each student must score at least 60 for the CLO average. If it has not fulfilled it, the student must undertake a repair assignment for the related CLO, except for CLO1.
