1. Course Identification
| Course | Organic Chemistry | |||
| Faculty | Mathematics and Natural Sciences | Study Program | Pharmacy | |
| Code | SFA-106 | Credits | 3 credits | |
| Group | Study Program | Status | Compulsory | |
| Semester | I (First) | Availability | Limited in Study Program | |
| Learning Method | Class | Media | online | |
| Cluster | Biomedical and Pharmaceutical Sciences: Pharmaceutical Chemistry | Prerequisite | – | |
2. Course Description
Mata kuliah Kimia Organik dalam Kurikulum Farmasi 2017 diberikan kepada mahasiswa semester I (satu) dengan bobot 3 sks. Mata kuliah ini merupakan mata kuliah wajib yang dapat ditempuh tanpa prasyarat. Mata Kuliah ini bertujuan untuk mendukung Capaian Pembelajaran Lulusan berupa kemampuan untuk berperilaku profesional dan etis serta pengetahuan dasar ilmu kefarmasian. Pada mata kuliah ini mahasiswa akan mempelajari tentang Struktur dan hibridisasi atom, jenis ikatan, jenis interaksi antar senyawa, stereokimia, asam basa berdasar struktur, sifat fisikokimia senyawa berdasarkan gugus fungsinya, struktur, sifat kimia dan geometri senyawa makromolekul, reaksi substitusi dan tautomeri keto-enol. Mahasiswa juga dibiasakan untuk bersikap jujur serta disiplin selama mengikuti perkuliahan ini.
1. Learning Outcome
| LO Code | LO |
| SIF3 | Able to carefully and thoroughly apply responsible attitude, dedication and punctuality based on sincerity, honesty and integrity at work along with the ability to show bravery in expressing the truth whilst maintaining politeness, ethics and affection and promoting mutual benefit. |
| PEF1 | Able to comprehend the theories, methods and concepts within the scope of pharmaceutical sciences and technologies in the disciplines of pharmaceutics, pharmacognosy, pharmaceutical chemistry and pharmacology as well as their application in pharmaceutical works. |
2. Course Learning Outcome and indicators
| Supported LO Code | CLO Code | CLO and indicators |
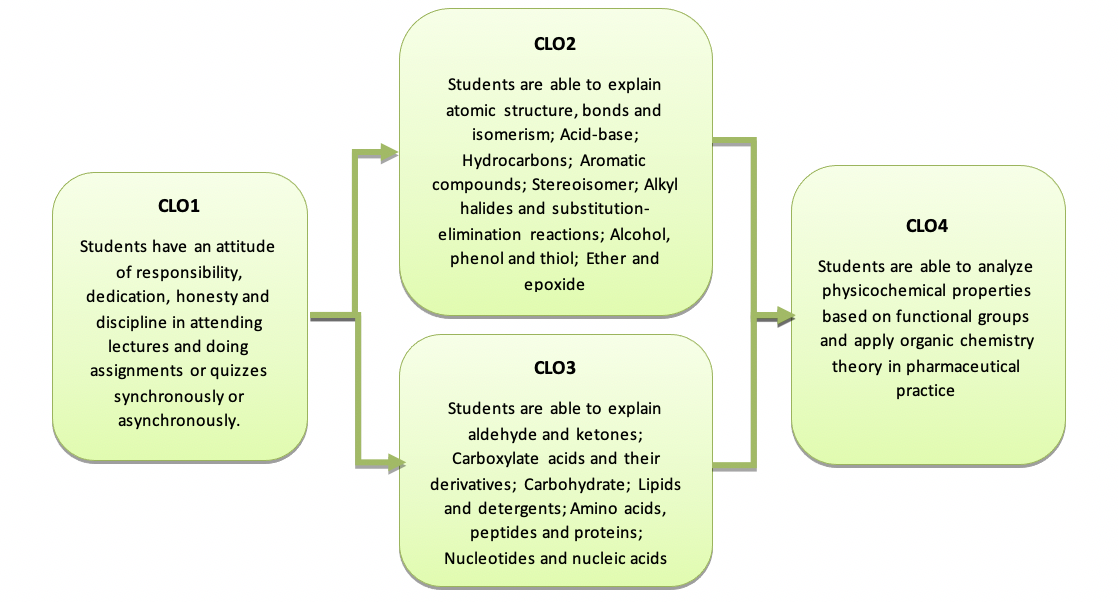
| SIF3 | CLO1 | Students have an attitude of responsibility, dedication, honesty and discipline in attending lectures and doing assignments or quizzes synchronously or asynchronously.
1. Students attend synchronous meetings and collect assignments according to a set schedule 2. Students take asynchronous lectures in a disciplined manner and carry out any accompanying activities completely and according to the time allocation provided. |
| PEF1 | CLO2 | Students are able to explain atomic structure, bonds and isomerism; Acid-base; Hydrocarbons; Aromatic compounds; Stereoisomer; Alkyl halides and substitution-elimination reactions; Alcohol, phenol and thiol; Ether and epoxide
1. Students are able to correctly explain atomic structure, bonds and isomerism; Acid-base; Hydrocarbons; Aromatic compounds; Stereoisomer; Alkyl halides and substitution-elimination reactions; Alcohol, phenol and thiol; Ether and epoxide |
| CLO3 | Students are able to explain aldehyde and ketones; Carboxylate compounds and their derivatives; Carbohydrate; Lipids and soaps; Amino acids, peptides and proteins; Nucleotides and nucleic acids
1. Students are able to correctly explain aldehyde and ketones; Carboxylate compounds and their derivatives; Carbohydrate; Lipids and soaps; Amino acids, peptides and proteins; Nucleotides and nucleic acids |
|
| CLO4 | Students are able to analyze physicochemical properties based on functional groups and apply organic chemistry theory in pharmaceutical practice
1. Students are able to correctly analyze physicochemical properties based on functional groups and correctly apply organic chemistry theory in pharmaceutical practice |
3. Course Learning Outcome Map Analysis
Primary References
- Hart DJ, Hadad CM, Craine LE, Hart H. 2012. Organic Chemistry: A Short Course 13th Ed., Brooks/Cole CENGAGE Learning, USA
- Winter A. 2008. Organic Chemistry I Workbook for Dummies. Wiley Publishing Inc. Canada
- Dewick PM. 2006. Essentials of Organic Chemistry for students of pharmacy, medicinal chemistry and biological chemistry. John Wiley and Sons Ltd. England
1. Learning activities
| Sesion | CLO | Topic | Learning activities |
| 1 | CLO2 | Introduction | Introduction from lecturer:
1. Learning contracts 2. Learning activities 3. Introduction of Marvin Sketch 4. Introduction of Organic Chemistry 5. Case discussion: importances of organic chemistry in Pharmacy |
| 2 | CLO2 | Atomic structure, bonds and isomerism | The lecturer explains about
1. How electrons are arranged in atoms 2. Ionic and covalent bonding 3. Valence 4. Isomerism 5. Structural fomulas 6. Classification according to functional groups Students independently practice working on problems |
| 3 | CLO2 | Hydrocarbons | The lecturer explains about
• Alkanes and Cycloalkanes • Alkenes and Alkynes Students independently practice working on problems |
| 4 | CLO2 | Aromatic compounds | Students study videos and slides (50 minutes)
Students do assignments (100 minutes) |
| 5 | CLO2 | Stereoisomer | The lecturer explains about
• Chirality and enantiomers • Configuration and the R-S convention • Stereochemistry Case: Thalidomide Students independently practice working on problems |
| 6 | CLO2 | Alcohol, phenol and thiol; Ether and epoxide | Students study videos and slides (50 minutes)
Students do assignments (100 minutes) |
| 7 | CLO2 | Alkyl halides and substitution-elimination reactions | The lecturer explains about
• Alkyl halides • Nucleophilic substitution • Elimination reaction Case: halogenated organic compounds from the sea Students independently practice working on problems |
| 8 | CLO2 | Midterm exam | Students do written questions (100 minutes). |
| 9 | CLO1
CLO3 CLO4 |
Aldehyde & Keton | The lecturer explains about aldehydes and ketones
• Nomenclature • The carbonyl groups • Keto-enol tautomerism • The acidity of α-hydrogens Students discuss the case given Case1: Aldehyde/ketone compounds in nature Students independently practice working on problems |
| 10 | CLO4 | Application of organic chemistry in pharmaceutical practices | Students study slides (30 minutes)
Students work on cases through group discussions and upload discussion results through classroom (120 minutes) |
| 11 | CLO3 | Carboxylic Acids and Their Derivatives | The lecturer explains about carboxylic acids and their derivatives
• Carboxylic acid nomenclature • Characteristics, acidity and α-hydrogen • Carboxylate derivatives and nomenclature • Reaction to salt formation and saponification Students discuss the case given Case2: Green chemistry in the synthesis of carboxylic acids Students independently practice working on problems |
| 12 | CLO1
CLO3 |
Carbohydrate | The lecturer explains about carbohydrates
• Definition and classification • Cyrillic monosaccharides • Cyclic hemiacetal structure • Pyranose and furanose • Formation of glycoside bonds Students discuss the case given Case3: Artificial fat from carbohydrates Students independently practice working on problems |
| 13 | CLO3
CLO4 |
Lipids and Detergents | Students study videos and slides (50 minutes)
Students do assignments (100 minutes) |
| 14 | CLO1
CLO3 |
Amino acid, peptide and protein | The lecturer explains about amino acids, peptides and proteins
Students independently practice working on problems |
| 15 | CLO1
CLO3 |
Nucleotide and nucleic acids | The lecturer explains about nucleotides and nucleic acids
Students independently practice working on problems |
| 16 | CLO3 | Final exam | Students do written questions (100 minutes). |
2. Learning Experience
| CLO Code | Learning Process |
| CLO1 | Synchronous and asynchronous online learning. Self-study using the Marvin Sketch and Google Form programs |
| CLO2 | Synchronous and asynchronous online learning. Self-study using the Marvin Sketch and Google Form programs |
| CLO3 | Synchronous and asynchronous online learning. Self-study using the Marvin Sketch and Google Form programs |
| CLO4 | Synchronous and asynchronous online learning. Group discussion to solve cases |
1. Assessment
| CLO Code | Assessment | Weight |
| CLO1 | Presence and liveliness on time submission of assignments
Independent learning activities |
10% |
| CLO2 | Assignment
Quiz Examination |
40% |
| CLO3 | Assignment
Quiz Examination |
35% |
| CLO4 | Assignments. | 15% |
2. Grading and Evaluation Systems
Grading system uses reference assessment as follows:
| Letter Value | Weight | Minimum Value | Value Range |
| A | 4 | 80 | 80.00 – 100 |
| A- | 3.75 | 77.5 | 77.50 – 79.99 |
| A/B | 3.5 | 75 | 75.00 – 77.49 |
| B+ | 3.25 | 72.5 | 72.50 – 74.99 |
| B | 3 | 70 | 70.00 – 72.49 |
| B- | 2.75 | 67.5 | 67.50 – 69.99 |
| B/C | 2.5 | 65 | 65.00 – 67.49 |
| C+ | 2.25 | 62.5 | 62.50 – 64.99 |
| C | 2 | 60 | 60.00 – 62.49 |
| C- | 1.75 | 55 | 55.00 – 59.99 |
| C/D | 1.5 | 50 | 50.00 – 54.99 |
| D+ | 1.25 | 45 | 45.00 – 49.99 |
| D | 1 | 40 | 40.00 – 44.99 |
| E | 0 | 0 | < 40 |
Evaluation Systems
Students must achieve a grade of at least 40 for average total course learning outcome. If it does not meet the requirements, they must carry out the test/remedial.
